We provide JH suite online (apkid: cz.jh.suite) in order to run this application in our online Android emulator.
Description:
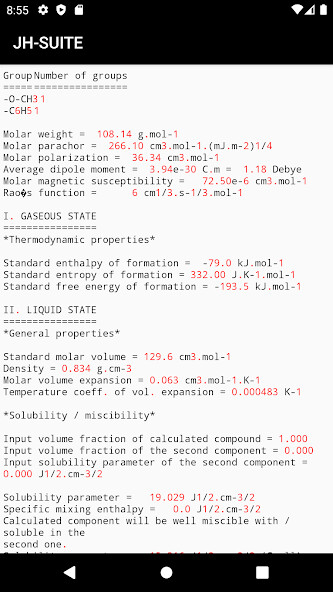
Run this app named JH suite using MyAndroid.
You can do it using our Android online emulator.
Alan Lika, Veronika Rikov, Ji Ludvk, 2017-2022
JH-suite (named according to Jaroslav Heyrovsk) is a set of small codes enabling fast empirical estimations.
Each individual program is written in X11-BASIC by Markus Hoffmann (https: //github.com/kollokollo/X11Basic) and can be edited by users according to their needs.
Before use, please read the corresponding manual in the Documents/jh-suite/doc folder.
Description of the individual codes:
Banks-Burnop - boiling points of organic compounds
Bayless - protonization constants for inorganic acids
Bayless-Ricci - protonization constants for inorganic acids
Bell - protonization constants for inorganic oxo acids
Brown I - complex formation equilibria, parametrized hard & soft acid-base principle, inorganic ligands
Brown II - complex formation equilibria, parametrized hard & soft acid-base principle, organic ligands: amines, carboxylic acids and amino acids
Brown III - precipitation equilibria, parametrized hard & soft acid-base principle, inorganic species
Brown IV - redox equilibria, parametrized hard & soft acid-base principle, inorganic species
Brown-Stein - melting and boiling points of organic compounds
Cabani - hydration Gibbs energy
Clifford I - precipitation equilibria, electronegativity calculation scheme
Clifford II - precipitation equilibria, electronegativity calculation scheme
Dick - equilibria among amino acids and peptides
Edwards I - complex formation equilibria, parametrized hard & soft acid-base principle
Edwards I - rate constants of nucleophilic substitution on Pt(II)-centre
Eigen-Fuoss I - complex formation equilibria, outer sphere aducts
Eigen-Fuoss II - rate constants of outer sphere nucleophilic substitutions in complexes
Fuoss - complex formation equilibria, ion pairs
Hammett I - organic acids protonization constants
Hammett II - selected organic reactions rate coefficients
Hancock I - complex formation equilibria, parametrized hard & soft acid-base principle
Hancock II - complex formation equilibria, parametrized hard & soft acid-base principle, generalized for both small and large donor atoms
Jankowski - formation Gibbs energy of (bio)organic solutes
Joback - basic organic thermodynamic estimations
Klopman I - solubility of organic compounds in water
Klopman II - solubility of organic compounds in water
Marcus - redox potentials of elementary reactions
Mavrovouniotis - standard Gibbs energy of aqueous organic solutes
Misono I - complex formation equilibria, parametrized hard & soft acid-base principle
Misono II - complex formation equilibria, parametrized hard & soft acid-base principle
Pauling I - protonization constants of inorganic oxo acids, empirical rules
Pauling II - standard enthalpy of fussion of any compound in its standard state
Pedley - standard enthalpy of fussion of organic compounds
PCM - Partial Charge Model, calculation of atomic charges in any species - molecules, ions - based on electronegativity equalization principle
Polymer - thermodynamic, spectroscopic, mechanical and other properties of organic compounds and polymers from group contributions
Ricci - protonization constants for inorganic oxo acids, empirical rules
Sanderson - thermodynamic properties and charge distribution based on one of the broadest empirical calculation scheme
Simamora - melting and boiling points of organic compounds where no hydrogen-bonds are employed
Yamada - complex formation equilibria, parametrized hard & soft acid-base principle
Yingst - complex formation equilibria, parametrized hard & soft acid-base principle
Zhou - rate constants of e.g.
acid-base reactions and other diffussion controlled processes
Zuman I-VII - polarographic half-wave potentials of organic compounds
JH-suite (named according to Jaroslav Heyrovsk) is a set of small codes enabling fast empirical estimations.
Each individual program is written in X11-BASIC by Markus Hoffmann (https: //github.com/kollokollo/X11Basic) and can be edited by users according to their needs.
Before use, please read the corresponding manual in the Documents/jh-suite/doc folder.
Description of the individual codes:
Banks-Burnop - boiling points of organic compounds
Bayless - protonization constants for inorganic acids
Bayless-Ricci - protonization constants for inorganic acids
Bell - protonization constants for inorganic oxo acids
Brown I - complex formation equilibria, parametrized hard & soft acid-base principle, inorganic ligands
Brown II - complex formation equilibria, parametrized hard & soft acid-base principle, organic ligands: amines, carboxylic acids and amino acids
Brown III - precipitation equilibria, parametrized hard & soft acid-base principle, inorganic species
Brown IV - redox equilibria, parametrized hard & soft acid-base principle, inorganic species
Brown-Stein - melting and boiling points of organic compounds
Cabani - hydration Gibbs energy
Clifford I - precipitation equilibria, electronegativity calculation scheme
Clifford II - precipitation equilibria, electronegativity calculation scheme
Dick - equilibria among amino acids and peptides
Edwards I - complex formation equilibria, parametrized hard & soft acid-base principle
Edwards I - rate constants of nucleophilic substitution on Pt(II)-centre
Eigen-Fuoss I - complex formation equilibria, outer sphere aducts
Eigen-Fuoss II - rate constants of outer sphere nucleophilic substitutions in complexes
Fuoss - complex formation equilibria, ion pairs
Hammett I - organic acids protonization constants
Hammett II - selected organic reactions rate coefficients
Hancock I - complex formation equilibria, parametrized hard & soft acid-base principle
Hancock II - complex formation equilibria, parametrized hard & soft acid-base principle, generalized for both small and large donor atoms
Jankowski - formation Gibbs energy of (bio)organic solutes
Joback - basic organic thermodynamic estimations
Klopman I - solubility of organic compounds in water
Klopman II - solubility of organic compounds in water
Marcus - redox potentials of elementary reactions
Mavrovouniotis - standard Gibbs energy of aqueous organic solutes
Misono I - complex formation equilibria, parametrized hard & soft acid-base principle
Misono II - complex formation equilibria, parametrized hard & soft acid-base principle
Pauling I - protonization constants of inorganic oxo acids, empirical rules
Pauling II - standard enthalpy of fussion of any compound in its standard state
Pedley - standard enthalpy of fussion of organic compounds
PCM - Partial Charge Model, calculation of atomic charges in any species - molecules, ions - based on electronegativity equalization principle
Polymer - thermodynamic, spectroscopic, mechanical and other properties of organic compounds and polymers from group contributions
Ricci - protonization constants for inorganic oxo acids, empirical rules
Sanderson - thermodynamic properties and charge distribution based on one of the broadest empirical calculation scheme
Simamora - melting and boiling points of organic compounds where no hydrogen-bonds are employed
Yamada - complex formation equilibria, parametrized hard & soft acid-base principle
Yingst - complex formation equilibria, parametrized hard & soft acid-base principle
Zhou - rate constants of e.g.
acid-base reactions and other diffussion controlled processes
Zuman I-VII - polarographic half-wave potentials of organic compounds
MyAndroid is not a downloader online for JH suite. It only allows to test online JH suite with apkid cz.jh.suite. MyAndroid provides the official Google Play Store to run JH suite online.
©2024. MyAndroid. All Rights Reserved.
By OffiDocs Group OU – Registry code: 1609791 -VAT number: EE102345621.